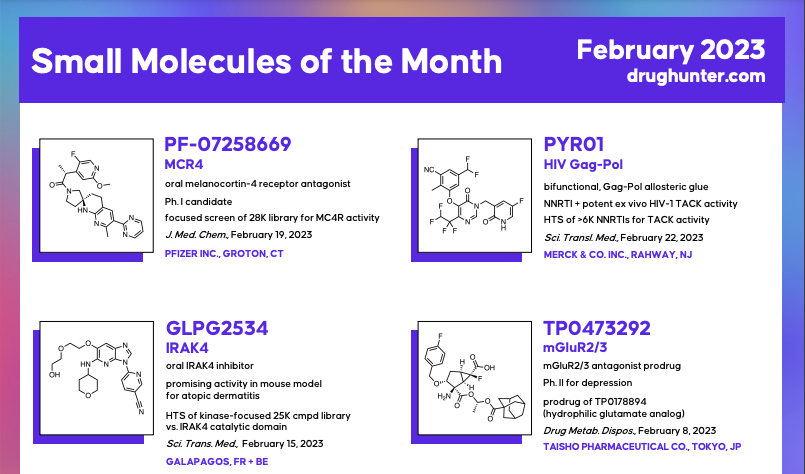
NOTICE: To support planned enhancements and preserve Drug Hunter's scientific independence from advertisers, the "Molecules of the Month" series will soon become a "Members Only" feature. If your institution is not already a member, you can request plan information here.
June was notable for several high-profile and structurally related WEE1 inhibitors, including AstraZeneca’s defunct adavosertib and Zentalis’ azenosertib, raising general concerns about the safety of this class of drugs. Biomea Fusion’s menin inhibitor also encountered a safety issue and was placed on a clinical hold. Concentric Analgesics’ first-in-class water-soluble prodrug and InnoCare Pharma’s brain-penetrant TRK (tropomyosin receptor kinase) inhibitor made our list as well. Other notable molecules from June include a next-generation dual EZH1/2 (enhancer of zeste homolog 1/2) inhibitor from Constellation Pharmaceuticals and an eIF2B (eukaryotic translation initiation factor 2B) activator from Calico Life Sciences and AbbVie for treating an ultra-rare progressive leukoencephalopathy.
You can find out what compounds made our June 2024 Molecules of the Month list and check out recent articles for each, linked below:
adavosertib – Ph. II trial (NCT03284385) results were published for the WEE1 inhibitor adavosertib in patients with solid tumors (cohort A) and clear cell renal cell carcinoma (ccRCC, cohort B) with pathogenic SETD2 mutations. Adavosertib, acquired by AstraZeneca from Merck in 2013, was administered orally at 300 mg QD on days 1-5 and 8-12 of each 21-day cycle. Among 18 patients enrolled, the median treatment duration was 1.28 months. No objective responses were observed, leading to halted accrual after stage 1. Minor tumor regressions occurred in 22% of patients, and stable disease (SD) in 56%, including three patients with SD > 4 months. Common adverse events included nausea, anemia, diarrhea, and neutropenia, with 50% experiencing Grade ≥ 3 events. These safety concerns are consistent with previously released Ph II trial (NCT04590248) findings in patients with recurrent or persistent uterine serous carcinoma, suggesting adavosertib is clinically active but not well-tolerated. Notably, while AstraZeneca halted the development of adavosertib in mid-2022, the FDA has just placed Zentalis Pharmaceuticals’ structurally related WEE1 inhibitor, azenosertib (ZN-c3), on a partial clinical hold following two patient deaths.
vocacapsaicin – A first-in-class, water-soluble prodrug that rapidly converts to capsaicin, a TRPV1-agonist, being developed by Concentric Analgesics for post-surgical pain. In a triple-blinded, randomized, placebo-controlled Ph. II trial (NCT03599089), vocacapsaicin administered intraoperatively reduced pain and decreased opioid consumption in the first 96 hours after bunionectomy, compared to placebo, with no local or systemic toxicity. Vocacapsaicin has also shown clinical efficacy in patients following total knee arthroplasty, abdominoplasty, and ventral hernia repair and is expected to progress to Ph. III for post-surgical pain.
zurletrectinib (ICP-723) – A potent, orally bioavailable, brain-penetrant next-generation TRK inhibitor from InnoCare Pharma with intracranial activity against NTRK fusion-positive tumors with on-target resistance to first-generation agents. Zurletrectinib is currently being evaluated in an open-label Ph. I/II trial (NCT04685226) in patients with advanced solid tumors.
senaparib (IMP4297) – Impact Therapeutics’ oral PARP1/2 (poly (ADP-ribose) polymerases 1/2) inhibitor with demonstrated efficacy in CDX and PDX models in tumors harboring BRCA1/2 mutations. Combination studies suggest that senaparib with temozolomide exhibits synergistic cytotoxicity in vitro and in vivo. A Ph. III clinical study for advanced ovarian cancer patients met its primary endpoint, and a new drug application has been accepted by China's National Medical Products Administration for review.
PF-06648671 – An oral, brain-penetrant GSM (γ-secretase modulator) discovered and developed by Pfizer for Alzheimer’s disease. Structurally notable is the 2,5-cis-tetrahydrofuran linker that imparts conformational rigidity and locks the compound into its bioactive conformation. PF-06648671 has excellent cell potency, physicochemical properties, favorable preclinical PK profile, and good rodent brain penetration, leading to robust PD. In January 2018, Pfizer announced it was discontinuing research and development in neurology, including PF-06648671.
fosigotifator (ABBV-CLS-7262) – Calico Life Science’s and AbbVie’s orally bioavailable eIF2B activator prodrug being developed as a potential treatment for vanishing white matter (VWM) disease, an ultra-rare progressive leukoencephalopathy. Accepted into the FDA’s START Pilot Program, fosigotifator is currently in an open-label Ph. I/II trial (NCT05757141) for adults and pediatrics with VWM disease and a randomized, double-blind Ph. I trial (NCT04948645) in patients with ALS.
tulmimetostat (CPI-0209) – An orally bioavailable, next-generation dual EZH1/2 inhibitor from Constellation Pharmaceuticals (MorphoSys AG, Novartis) with demonstrated efficacy in multiple ARID1A mutant bladder, ovarian, and endometrial tumor models and enhanced cisplatin response in chemotherapy-resistant models. Interim Ph. I (NCT05942300) clinical data indicates tulmimetostat has durable exposure, strong target engagement, and the potential to achieve clinical benefit in solid tumors as a monotherapy, as well as in combination with carboplatin.
BMF-219 – A potent, orally bioavailable, and selective covalent inhibitor of menin, a histone lysine methyltransferase, from Biomea Fusion being developed for type 1 (NCT06152042) and type 2 (NCT05731544) diabetes mellitus that has now been placed on a full clinical hold. The FDA cited liver enzyme elevations indicative of possible drug-induced hepatotoxicity as the reason for the clinical hold. BMF-219 is also being developed for the treatment of multiple myeloma, leukemia, lymphoma, and KRAS-driven non-small-cell lung cancer, pancreatic cancer, and colorectal cancer.
sabizabulin (VERU-111) – A potent, orally bioavailable, and well-tolerated tubulin inhibitor currently being developed by Veru. Mechanistically, sabizabulin disrupts microtubule formation inducing mitotic catastrophe leading to apoptosis in a concentration-dependent manner. Notably, it suppressed ovarian tumor growth and completely suppressed organ metastasis in vivo, and has comparable efficacy to paclitaxel, the current first-line treatment for ovarian cancer. Sabizabulin has advanced to a Ph. II trial (NCT05079360) for ER+HER2- metastatic breast cancer, a Ph. III trial (NCT04844749) for metastatic castration-resistant prostate cancer, and a Ph. III trial (NCT04842747) for the treatment of COVID-19.
XRD-0394 – A potent, selective, and orally bioavailable dual inhibitor of two DNA damage response kinases, ATM (ataxia telangiectasia mutated) kinase and DNA-PK (deoxyribonucleic acid-dependent protein kinase) from XRad Therapeutics with significantly enhanced tumor cell killing in the context of therapeutic ionizing irradiation in vitro and in vivo. XRD-0394 potentiates the effectiveness of topoisomerase I inhibitors in vitro and shows single-agent activity and synergy in combination with PARP inhibitors in cells lacking BRCA1/2. A Ph. I trial (NCT05002140) with XRD-0394 in combination with radiotherapy has been completed in advanced cancer patients.
Check out our Curated Molecule Database Search tool, which enables you to uncover industry trends in therapeutic areas, target selection, and chemical matter, to inspire your drug discovery campaign.
Comments?
Want to share your scientific insight or contribute to the conversation about these molecules? Find out how here.
Sign up for our free weekly newsletter here to get more content like this straight to your inbox.