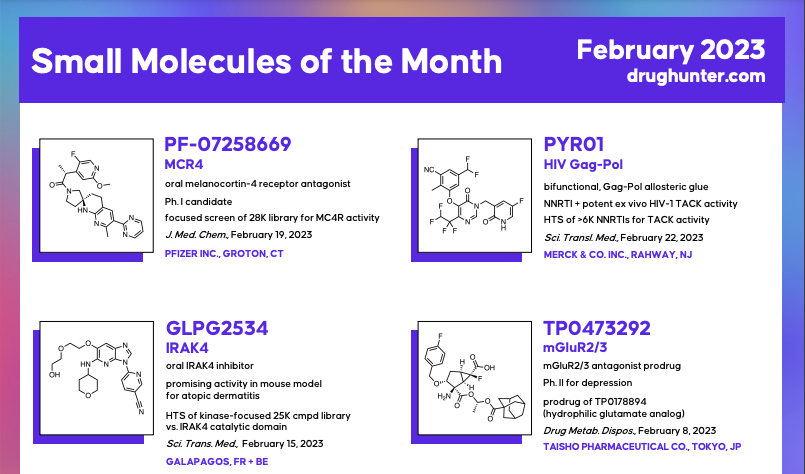
A Ph. IIb TYK2 inhibitor from Nimbus (just bought by Takeda) that may be more efficacious than deucravacitinib, the first and only approved CGRP receptor antagonist nasal spray for the treatment of acute migraines from BMS/Pfizer, a pomalidomide-derived Ph. I/Ib oral, IKZF2-selective molecular glue degrader for the treatment of advanced solid tumors from Novartis are just some of the examples for this month's MOTM. Learn more about the molecules by navigating to the full reviews on the right (when released) which cover: why the molecules matter, how they were discovered, the key steps behind optimization, how they work, why the targets were chosen, any interesting toxicology and clinical data, and more. In the meantime, as we release the full reviews throughout this month, you can find the article links that inspired us to write up these stories below:
TAK-279 - a Ph. Ilb oral, once-daily allosteric TYK2 inhibitor from Nimbus/Takeda
zavegepant - the first and only approved CGRP receptor antagonist nasal spray from BMS/Pfizer
revumenib - a Ph. I/II oral menin-KMT2A inhibitor from Vitae/Syndax
zunsemetinib - a Ph. IIa oral MK2 inhibitor from Confluence/Aclaris Therapeutics
JNJ-1802 - a Ph. I oral first-in-class DENV (NS3-NS4B) inhibitor from Janssen
nirogacestat - a Ph. III oral gamma secretase inhibitor from Pfizer/SpringWorks
linvencorvir - a Ph. II oral allosteric HBV modulator from Roche Shanghai
KW-6356 - a Ph. II oral A2A receptor antagonist/inverse agonist from Kyowa Kirin
DKY709 - a Ph. I/Ib oral IKZF2-selective molecular glue degrader from Novartis
ACT-777991 - a Ph. I oral reversible CXCR3 antagonist from Idorsia Pharmaceuticals
Sign up for our free weekly newsletter here to get more content like this straight to your inbox. Not a member? Get a free trial to check out substructure search and more features.